Calcium Atom on a White Background Stock Illustration Illustration of
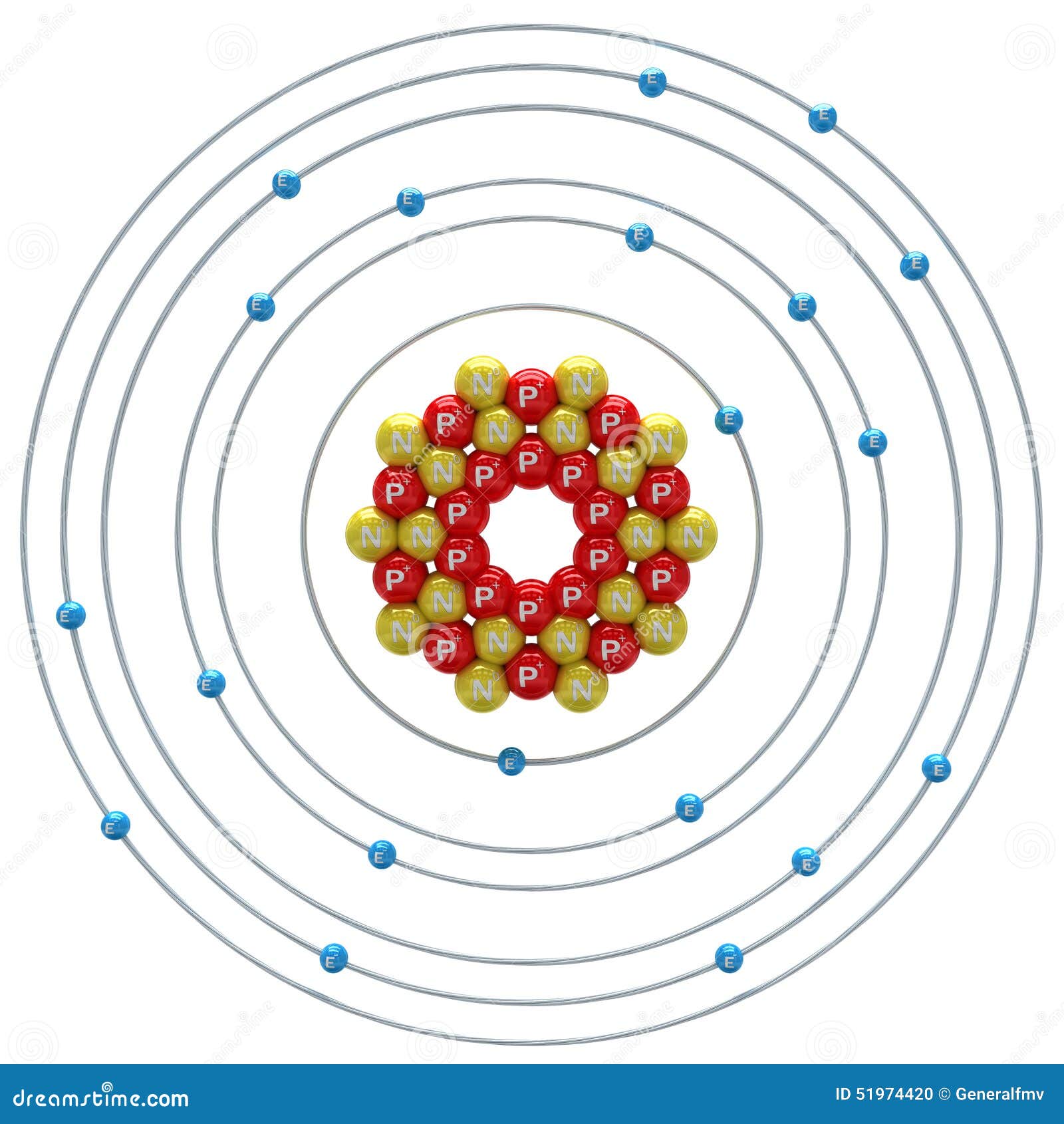
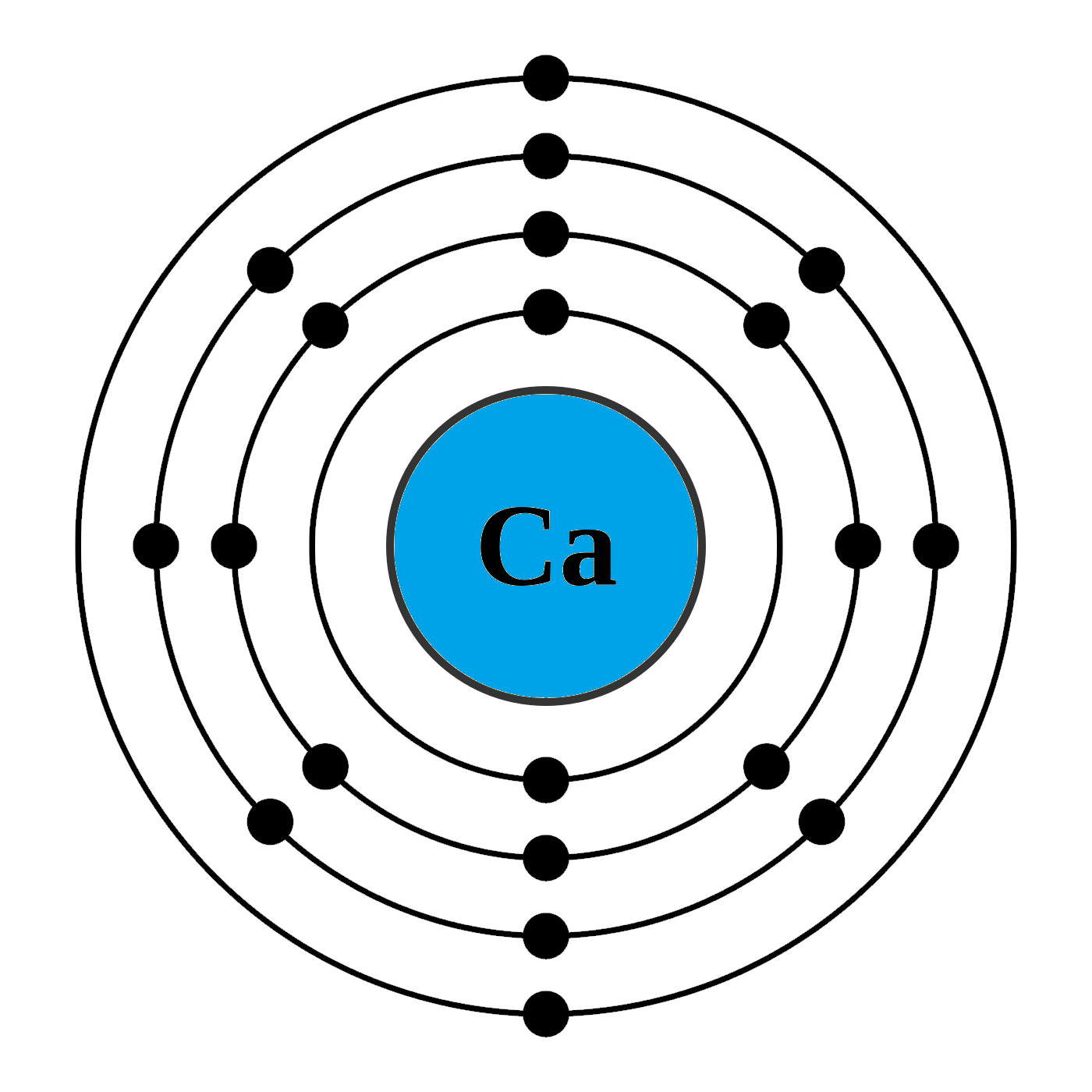
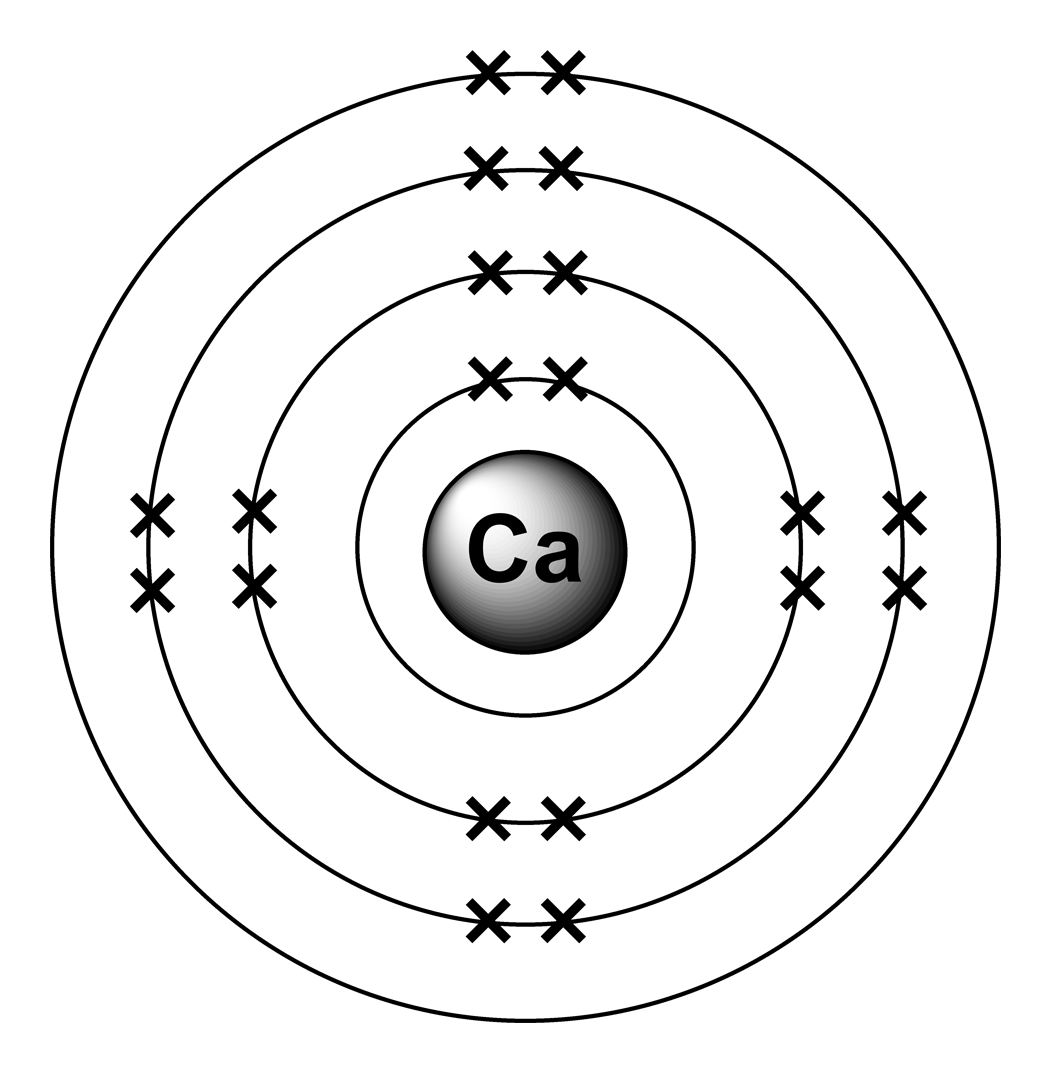
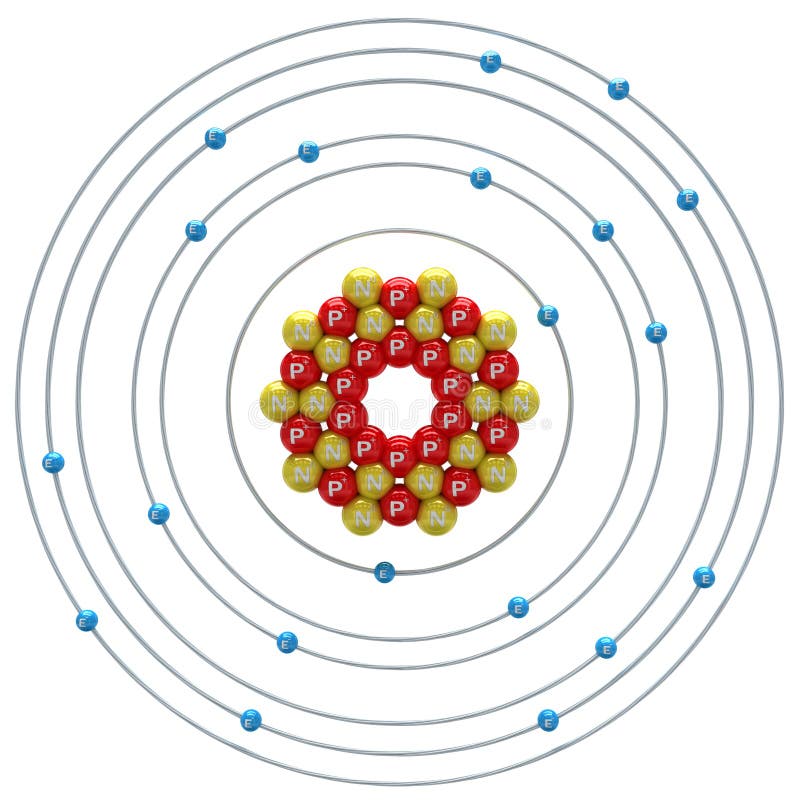
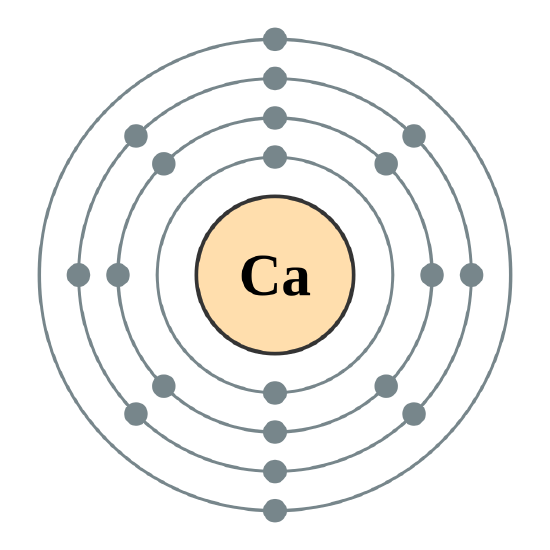
1st shell can hold 2 electrons. 2nd shell can hold 8 electrons. 3rd shell can hold 18 electrons. 4th shell can hold 32 electrons. Now the atomic number of calcium (Ca) is 20. Hence the electron arrangement in calcium is 2, 8, 8, 2. And the electron configuration of calcium is 1s2 2s2 2p6 3s2 3p6 4s2.

Bohr Model Representation Calcium Atom Number Stock Vector (Royalty
Bohr model of calcium: (CC BY-SA 2.0 uk;Greg Robson): Answer b. Bohr model of sulfur: (CC BY-SA 2.0 uk; Greg Robson). Valence electrons are located in the highest energy level of an atom. When drawing a Bohr diagram, the valence electrons would be present in the outermost electronic level/shell (furthest away from the nucleus). An atom can have.
How can I draw electronic configuration of calcium in a shell nxwe70dd
Bohr model is a structural model in which the negatively charged electrons revolve around the positively charged nucleus. This is similar to the planets revolving around the sun, except that the orbits are non-planar. The electrons move in a fixed orbits (shells) and each orbit has a fixed energy. Each orbit (or shell) can hold a certain number.
Bohr Diagram Of Calcium
The electron volt (eV) is a convenient unit of energy for expressing atomic-scale energies. It is the amount of energy that an electron gains when subjected to a potential of 1 volt; 1 eV = 1.602 ×10−19 J 1 e V = 1.602 × 10 − 19 J. Using the Bohr model, determine the energy, in electron volts, of the photon produced when an electron in a.
Calcium Atom on a White Background Stock Illustration Illustration of
Bohr model of the atom In the Bohr model of the atom, electrons travel in defined circular orbits around the nucleus. The orbits are labeled by an integer, the quantum number n. Electrons can jump from one orbit to another by emitting or absorbing energy.

Calcium atomic properties 141 images, photos et images vectorielles
The Bohr Model is a modification of an earlier atomic model, the Rutherford Model. The Bohr Model has an atom with a positively-charged nucleus surrounded by negatively-charged electrons that have circular, planetary-like orbits. Today, we know that the Bohr Model has some inaccuracies, but it's still used because of its simple approach to.
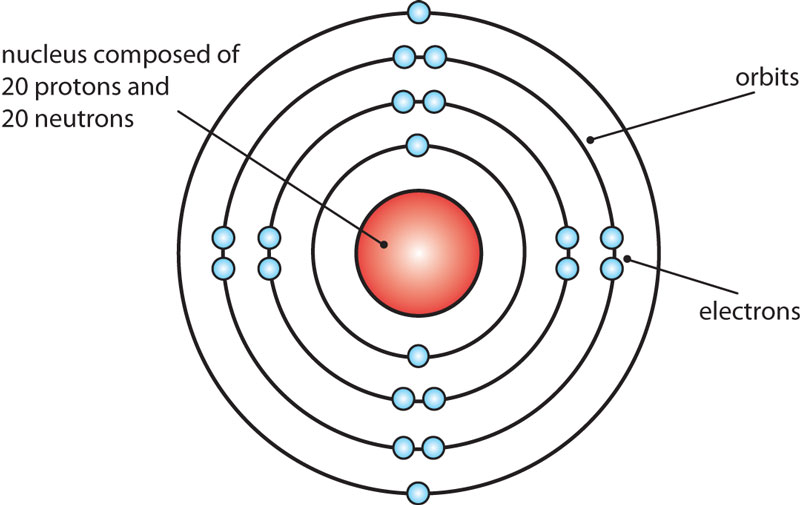
Calcium Bohr diagram Calcium
The Bohr model of the hydrogen atom explains the connection between the quantization of photons and the quantized emission from atoms. Bohr described the hydrogen atom in terms of an electron moving in a circular orbit about a nucleus.. Suggest a reason for the observation that the spectrum of calcium is more complicated than the spectrum of.
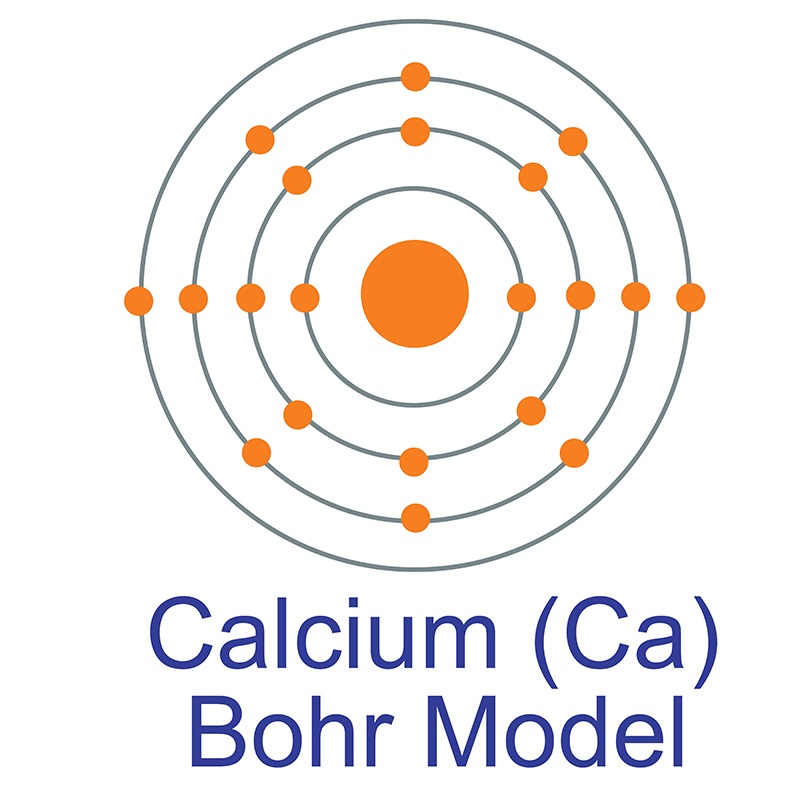
Calcium (Ca) AMERICAN ELEMENTS
Bohr model of all Elements is mentioned in the chart below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2).
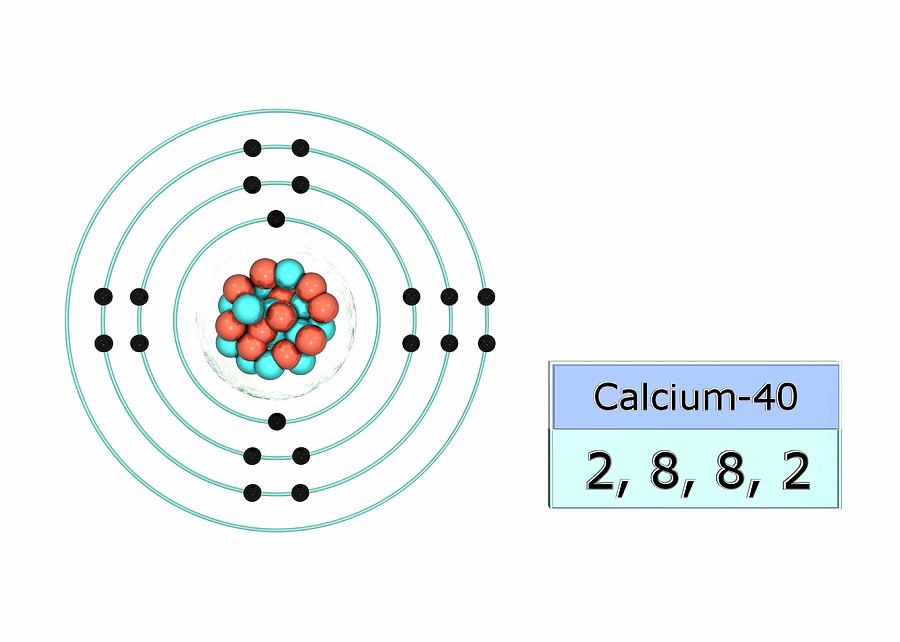
Calcium Electron Dot Diagram Photos Cantik
The Bohr model of the hydrogen atom explains the connection between the quantization of photons and the quantized emission from atoms. Bohr described the hydrogen atom in terms of an electron moving in a circular orbit about a nucleus. He postulated that the electron was restricted to certain orbits characterized by discrete energies.
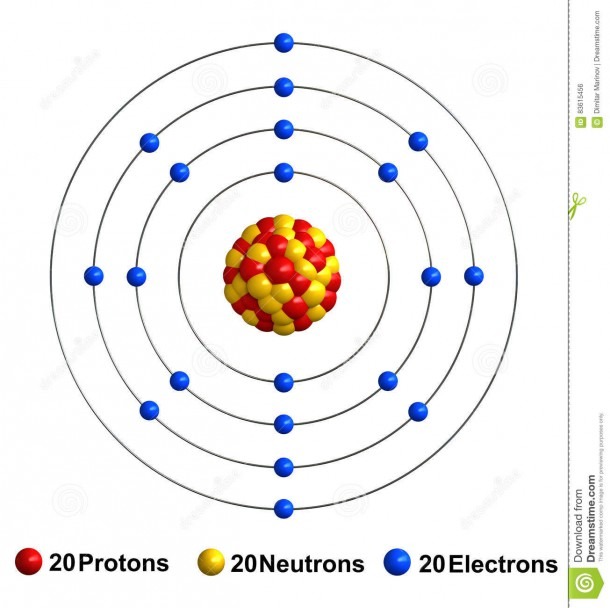
Bohr Diagram Of Calcium
Course: Class 9 Chemistry (India) > Unit 4. Lesson 1: Models of an atom. Discovery of the electron and nucleus. Rutherford's gold foil experiment. Drawback of the Rutherford model. Bohr's model of an atom. Atomic structure. Science >. Class 9 Chemistry (India) >.
3.9 Atomic Models of the Twentieth Century Chemistry LibreTexts
The Bohr model of the hydrogen atom ( Z = 1) or a hydrogen-like ion ( Z > 1 ), where the negatively charged electron confined to an atomic shell encircles a small, positively charged atomic nucleus and where an electron jumps between orbits, is accompanied by an emitted or absorbed amount of electromagnetic energy ( hν ). [1]

Calcium atom bohr model Royalty Free Vector Image
The Bohr model Google Classroom Learn how Bohr models are used to represent atoms. Atoms are way too small to see with the naked eye (and even most microscopes). So, we represent atoms using models. Models help us visualize atomic structure. They also help us explain and predict the behavior of atoms.
Bohr Model Atom Electron Configuration Argon Calcium Molecular
9.4: The Bohr Model - Atoms with Orbits is shared under a CK-12 license and was authored, remixed, and/or curated by Marisa Alviar-Agnew & Henry Agnew. LICENSED UNDER. Bohr's model suggests that each atom has a set of unchangeable energy levels, and electrons in the electron cloud of that atom must be in one of those energy levels.

whats the lewis dot diagram for calcium
Steps to draw the Bohr Model of Calcium atom 1. Find the number of protons, electrons, and neutrons in the Calcium Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei. Electrons are the negatively charged particles that orbit the nucleus of an atom

How to Build a Model of a Calcium Atom Articles MerchantCircle
Calcium has 2 electrons in its first shell, 8 in its second, 8 in its third, and 2 in its fourth.Check me out: http://www.chemistnate.com

Calcium, atomic structure Stock Image C018/3701 Science Photo Library
Atomic Structure (Bohr Model) for Calcium (Ca) Wayne Breslyn 722K subscribers Join Subscribe Subscribed 84 10K views 1 year ago In this video we'll look at the atomic structure and Bohr.